Chloromag Prescribing Information
Package insert / product label
Generic name: magnesium chloride
Dosage form: injection, solution
Drug class: Minerals and electrolytes
Medically reviewed by Drugs.com. Last updated on Mar 1, 2024.
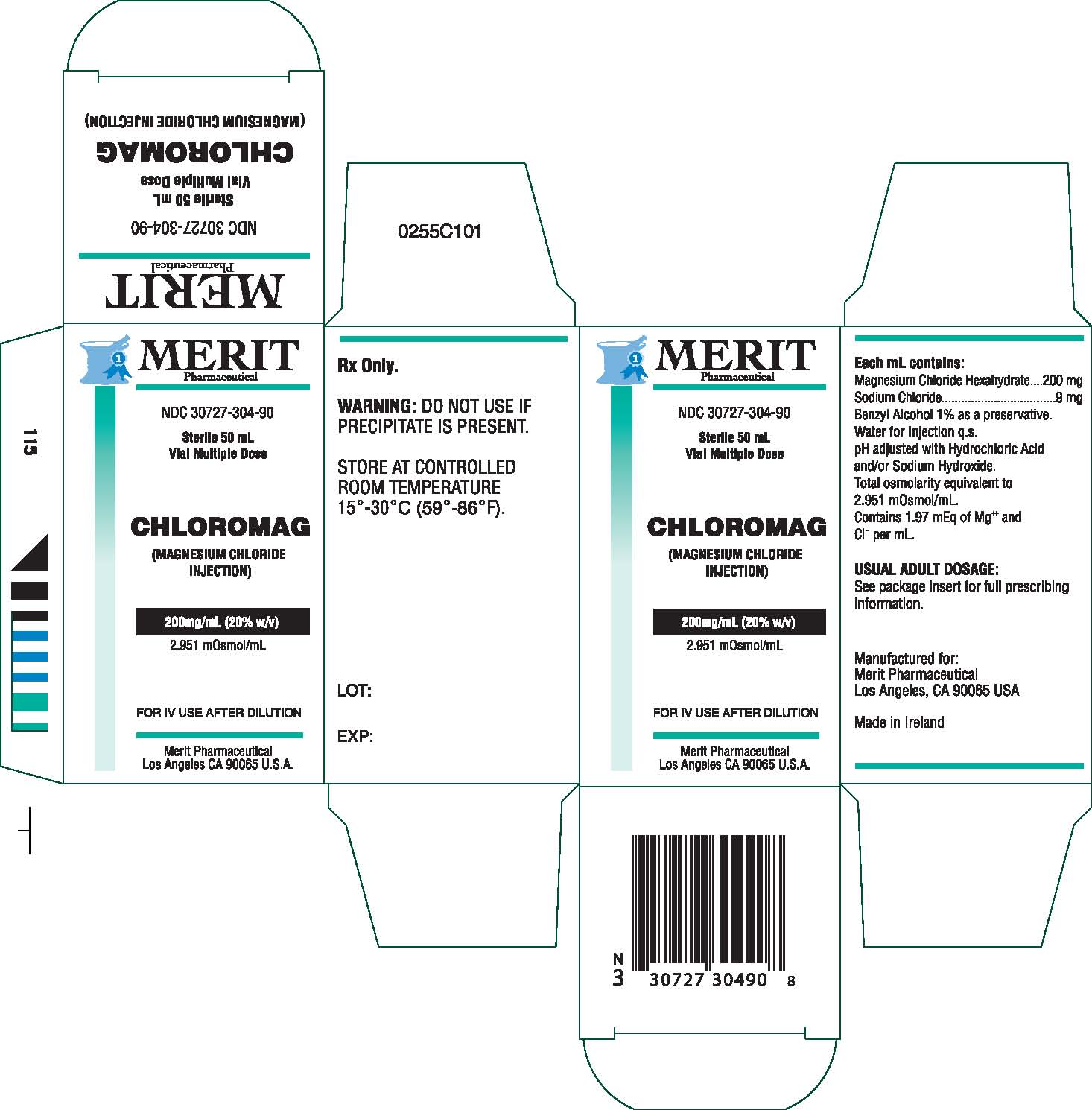
Chloromag Description
Magnesium Chloride Injection is a sterile solution of
Magnesium Chloride Hexahydrate in Water for Injection
q.s. Each mL contains Magnesium Chloride Hexahydrate
200 mg, Sodium Chloride 9 mg, Benzyl Alcohol 1% as
a preservative, Water for Injection, q.s. pH adjusted
with Hydrochloric Acid and/or Sodium Hydroxide. Total
osmolarity equivalent to 2951 mOsm/L.
Contains 1.97 mEq of Mg++ and Cl- per mL.
The structural formula is MgCl2•6H2O.
ACTIONS
Magnesium is the second most plentiful cation within
cellular fluids. It is an important activator of many
enzyme systems and deficits are accompanied by a
variety of functional disturbances.
Indications and Usage for Chloromag
As an electrolyte replenisher in magnesium
deficiencies.
Contraindications
Magnesium Chloride Injection should not be administered
if there is renal impairment, marked myocardial disease
or to comatose patients.
Warnings
Do not use if a precipitate is present.
Related/similar drugs
lisinopril, metoprolol, aspirin, propranolol, Plavix, Brilinta, magnesium oxide
Precautions
The usual precautions for parenteral administration
should be observed. Administer with caution if flushing
and sweating occurs. A preparation of a calcium salt
should be readily available for intravenous injection
to counteract potential serious signs of magnesium
intoxication. As long as deep tendon reflexes are active
it is probable that the patient will not develop respiratory
paralysis. Respiration and blood pressure should be
carefully observed during and after administration of
Magnesium Chloride Injection.
CHLOROMAG
magnesium chloride injection, solution |
|
|
|
|
|
|
|
|
|
|
|
|
More about Chloromag (magnesium chloride)
Patient resources
Professional resources
Related treatment guides
Medical Disclaimer